Abstract
Introduction: Epstein-Bar-Virus (EBV) associated post-transplant lymphoproliferative disorder (PTLD) is associated with increased mortality and morbidity in solid organ and hemopoietic-stem-cell (HSC) transplant recipients. Conventionally, it was being treated with reduction in immune suppressants, rituximab (Rx), chemotherapy, or radiotherapy. However, given the relapse-refractory (RR) nature of the disease, other agents such as EBV specific cytotoxic T-lymphocyte (EBV-CTL), brentuximab are being used with some success. In this review, we will assess the efficacy of such agents in PTLD.
Method: We performed literature search from 3 databases (PubMed, Embase and Web of Science) using the MeSH terms and keywords ''Post Transplant Lymphoproliferative Disorder'' and ''Treatment Outcome''. After excluding the duplicates, we conducted primary and secondary screening on 1564 studies, and finalized 9 studies for the meta-analysis (4 prospective studies, 2 retrospective studies, and 3 phase I/II clinical trials). Subgroup analysis was performed by using the OpenMetaAnalyst software, and quality evaluation was performed with the help of NIH quality assessment tool.
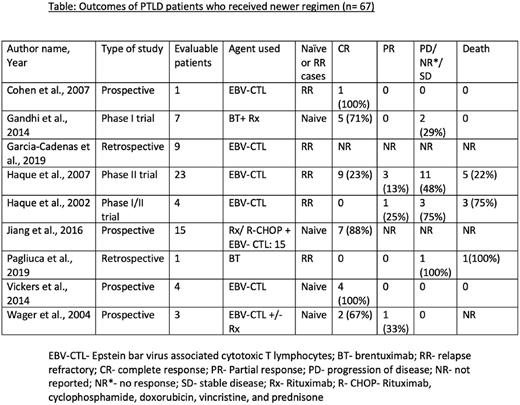
Results: We conducted meta-analysis on 67 patients from 9 studies, of which 29 (43.3%) were treatment-naïve, and 38 (57%) were RR. 59 (88%) received EBV-CTL, and 8 (12%) received brentuximab, either as a monotherapy in treatment-failure cases or as a combination therapy in naïve cases. For the EBV-CTL subgroup, the pooled complete response (CR) was 60.4% (95% CI 0.290-0.886, I²= 59.41%, p=0.031), pooled partial response (PR) was 10% (95% CI 0.002-0.270, I²= 0%, p=0.833), and pooled mortality was 24% (95% CI 0.026-0.534, I²= 30.3%, p=0.230). For brentuximab subgroup, the pooled CR was 55.2% (95% CI 0.118-0.986, I²= 42.98%, p=0.185), and pooled mortality was 34.3% (95% CI 0.165-0.537, I²= 58.94%, p=0.012). The common adverse effects seen from these therapies are graft-versus-host-disease and infection, however, given the heterogeneity, we could not perform pooled analysis.
Conclusion: There is no standard guideline available to treat RR PTLD. We conclude that CTL and brentuximab could be an appropriate second-line therapy in treatment failure cases or could be used concomitantly with rituximab for better patient outcome. Clinical trials with longer follow ups need to be conducted to assess safety and efficacy of these agents.
Disclosures
Anwer:BMS: Consultancy, Research Funding, Speakers Bureau; Allogene Therapeutics: Research Funding; Janssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal